
If the isotope ratio is reversed, the average atomic mass of chlorine will bea)35.0 ub)35.5 uc)36.0 ud)36.5 uCorrect answer is option 'D'. Can you explain this answer? theory, EduRev gives you anĪmple number of questions to practice 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. Can you explain this answer? has been provided alongside types of 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. Can you explain this answer?, a detailed solution for 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. Besides giving the explanation ofģ5Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively.

Can you explain this answer? defined & explained in the simplest way possible. Here you can find the meaning of 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. Can you explain this answer? covers all topics & solutions for ClExam.įind important definitions, questions, meanings, examples, exercises and tests below for 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. Information about 35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. The Question and answers have been prepared Can you explain this answer? for Clis part of Class 10 preparation. The average mass of the naturally occurring isotopes of an element in comparison to the mass of an atom of 12C is called relative atomic mass.Īs a result, the atomic mass of chlorine is 35.5u rather than a whole number like 36u or 35u.35Cl and 37Cl are the two isotopes of chlorine, in the ratio 3 : 1 respectively. The sum of the masses of protons, neutrons, and electrons in an atom or group of atoms is called atomic mass. It happens roughly a quarter of the time in nature.

There are 17 protons and 20 neutrons in the universe. Because both isotopes of chlorine have 17 positive protons in the nucleus and 17 negative protons from electrons in the surrounding orbitals, it doesn’t matter which isotope is present in a chemical reaction.Ĭonsider a chlorine atom, which has 17 protons and has two isotopes: chlorine 35 and chlorine 37. Relative atomic mass = ( 75 100 35) + ( 25 100 37) =35.5īecause neutrons have no charge, all isotopes of any element act chemically the same. The atomic weight, or relative atomic mass, is a weighted average of the weights of all chlorine isotopes. When the mass of chlorine is determined with a mass spectrometer, the result is 35.5. That means there is 75 percent Chlorine-35 and 25% Chlorine-37 in any pure chlorine mixture that can be isolated from all other elements.
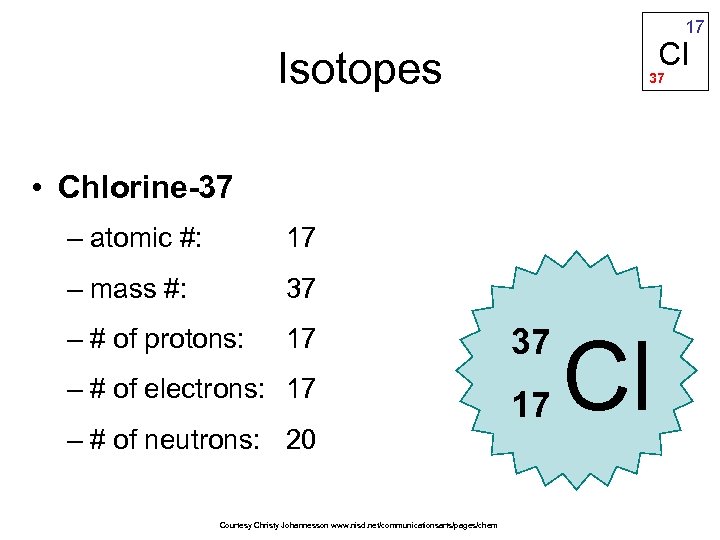
Chlorine-37 has 17 protons and 20 neutrons and is found about 25% of the time in nature. Chlorine comes in two forms: chlorine-35 and chlorine-37.Ĭhlorine-35, which has 17 protons and 18 neutrons, is found in nature roughly 75% of the time. Isotopes are atoms that have the same number of protons (17 in the case of chlorine) but variable numbers of neutrons. Why is the atomic mass of chlorine taken as 35.5 u and not a whole number like 35 u or 36 u explain?īecause of something called isotopes, chlorine has an atomic weight of 35.5 instead of 35.


 0 kommentar(er)
0 kommentar(er)
